
Time:2024-05-07 Read:2946
Photodynamic therapy (PDT) is an emerging therapeutic modality that harnesses the interaction between photosensitizers, light, and molecular oxygen to selectively destroy cancer cells. However, the current application of PDT is primarily focused on treating superficial or shallow tissue tumors. This restriction stems from the fact that the photosensitizers of traditional PDT predominantly respond to visible light, which has poor penetration into deep tissues due the absorption of most biomolecules, thereby limiting its efficacy in treating deep-seated tumors. To achieve greater light penetration depth, the near-infrared (NIR) light has emerged as an ideal option due to its lower absorption by most biological tissues within the NIR window (700-1700 nm). NIR light possesses enhanced tissue-penetrating capabilities, allowing for more efficient penetration of deep-seated tumors and activation of photosensitizers, thus improving the effectiveness of PDT. The combination of NIR light with suited photosensitizers enables deeper eradication of cancer cells and reduces damage to surrounding normal tissues.
To overcome the limitations of light penetration in PDT, upconversion nanoparticles (UCNPs) have been extensively investigated as promising candidates. Researchers have synthesized UCNPs with various properties by controlling their composition, structure, and surface modifications to further optimize their performance. For instance, doping with rare-earth ions such as erbium, gallium, and yttrium enables different upconversion spectra ranging from near-infrared to visible light. Additionally, factors such as the size, shape, and coating materials of the nanocrystals can influence the performance of UCNPs, including upconversion efficiency, photostability, and biocompatibility. Besides, UCNPs have demonstrated broad application prospects not only as light sources in PDT but also in areas such as biological imaging, drug release, and photothermal therapy. In PDT treatment, UCNPs convert near-infrared (NIR) light into higher-energy visible or ultraviolet light, enabling deeper tissue penetration and activation of photosensitizers at their optimal wavelengths for therapeutic effects. Additionally, UCNPs synthesized with precision can attain energy transfer efficiencies near 60%, ensuring effective energy conversion even in deep tissues. This makes UCNPs an ideal platform for enhancing the efficacy of PDT. Furthermore, the integration of UCNPs with photosensitizers is crucial as it directly influences the efficiency of reactive oxygen species (ROS) generation. Among various photosensitizers, protoporphyrin (PPIX) has garnered significant attention due to its favorable photophysical properties, biocompatibility, and clinical applicability. However, existing studies faced challenges such as limited and unstable photosensitizer loading of UCNPs, making it difficult to achieve sufficient ROS concentrations for effective tumor destruction, despite achieving enhanced light penetration depth. Meanwhile, the UCNPs cannot be actively enriched in the tumor site due to the lack of specific recognition ability. The primary challenge in cancer therapy is the disruption of the balance between cancerous and normal tissues during treatment. Due to physiological and microenvironmental differences between tumor and normal tissues, an increasing number of materials are being developed for targeted tumor therapy. Folic acid is widely used by researchers due to its affordability, accessibility, non-toxicity, non-immunogenicity, and high affinity to folate receptors. Based on this, seeking for a more efficient method in PDT, that the UCNPs with abundant photosensitizers can specifically gather into tumor tissues to generate a lot of ROS and then improve tumor clearance rates in deep-seated.
In this study, a novel composite material, PPIX-PEI-UCNP@FA NPs, was synthesized, comprising PPIX molecules, UCNPs doped with ytterbium (Yb) and erbium (Er), PEI, and FA. Current modifications of UCNPs with PEI typically involve a surface coating layer of PEI, which does not exploit the potential spatial structure provided by PEI to achieve higher drug loading and enhanced light absorption efficiency. In our work, the highly branched PEI rich in amino groups was used to link surface bifunctionalized UCNPs, forming a three-dimensional structure with a high specific surface area, centered around PPIX-loaded UCNP nodes. This architecture achieved a porphyrin loading rate of up to 0.69 wt.% through triple PPIX conjunction, while effectively reducing PEI toxicity by utilizing amine groups. The UCNPs extended the excitation wavelength to 980 nm, allowing therapeutic penetration beyond 10 mm. The branched PEI enhanced the loading efficiency of PPIX, leading to effective singlet oxygen generation and high tumor cell kill rates. The functionalization with folic acid (FA) enabled targeted drug delivery to cancer cells. The PPIX-PEI-UCNP@FA nanoparticles demonstrated properties of near-infrared excitation, deep-tissue penetration, high ROS production, and tumor-specific targeting. Compared to PPIX loaded PLGA-PEG NPs, our material achieves a 3-fold increase in drug loading, lower toxicity, and nearly triples ROS production in deep tissue. Benefiting from the high loading capacity offered by the spatial structure of PEI, our approach has doubled the targeting efficiency compared to systems without PEI. In vivo, after intravenous injection, the material binds to FA receptors on tumor cell membranes, internalizes, and, upon 980 nm laser exposure, generates ROS in deep-seated tumors. This study's findings are crucial for advancing PDT, overcoming traditional limitations, and enhancing clinical outcomes in cancer treatmen. Administered intravenously in mice, the NPs bind to tumor cell membrane FA receptors and internalizes, acting within mitochondria. Upon 980 nm laser exposure, it generates excessive ROS in deep-seated tumors, impairing mitochondrial function, decreasing MMP, and reducing ATP production. Activation of apoptosis-associated proteins via the mitochondrial-dependent pathway leads to the destruction of tumor cells. These findings are crucial for developing advanced PDT strategies, overcoming traditional limitations, and enhancing clinical outcomes in cancer treatment.
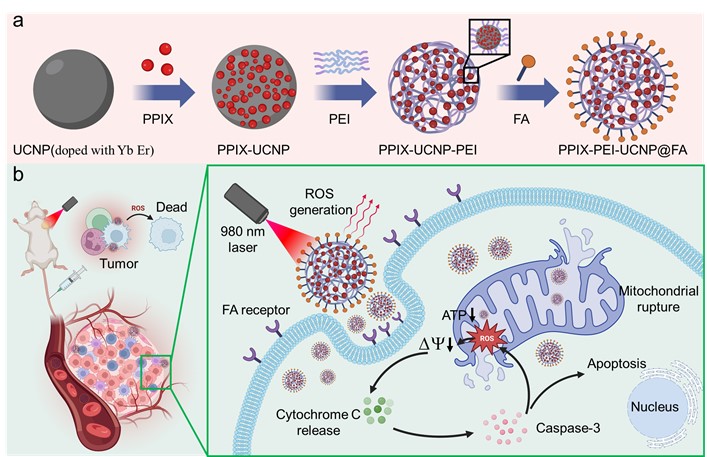
Figure (a) Scheme of synthesis of PPIX-PEI-UCNP@FA NPs. (b) Mechanism of PPIX-PEI-UCNP@FA NPs as PDT agent for tumor therapy via 980 nm laser.
The research was published in “Hongrui Shan, Xueqian Wang, Qiheng Wei, Hailang Dai, and Xianfeng Chen, Enriched endogenous photosensitizer for deep-seated tumors photodynamic therapy, Photonics Research, 12(5), 1024-1035 (2024)”.
Link: https://opg.optica.org/prj/fulltext.cfm?uri=prj-12-5-1024&id=549486